Volume: 22 Issue: 8 August 2024
FULL TEXT
The use of marginal donor livers, particularly steatotic livers, could help to resolve the problem of organ shortage and wait list mortality. Ischemia-free liver transplant with the potential to avoid ischemia-reperfusion injury and related complications, parti-cularly early allograft dysfunction, could positively encourage the use of marginal donor livers and extend the donor pool. Here, we describe the first case in a Western country of ischemia-free liver transplant of a marginal donor liver. To date, a research team in China is the only group to have described and used this technique. The technical and setup aspects are illustrated, and present controversies are discussed. A 58-year-old female patient received a transplant of a >60% steatotic donor liver. The transplant was accomplished with the ischemia-free liver transplant technique, and the donor liver was procured and transplanted under continuous normothermic machine perfusion. The donor liver functional para-meters under normothermic machine perfusion were reassuring, and recipient recovery was uneventful. Although ischemia-free liver transplant is a technically and organizationally demanding procedure, our case demonstrates the feasibility of the ischemia-free liver transplant technique and encourages the develop-ment and expansion of its use.
Key words : Early graft dysfunction, Extended donor criteria, Ischemia-free liver transplantation, Ischemia-reperfusion injury, Nonalcoholic fatty liver disease
Introduction
The ischemia-free liver transplant (IFLT) technique, first reported from China in 2017 by He and colleagues,1 greatly affected what transplant surgeons considered possible because of its potential to avoid ischemia-reperfusion injury (IRI). Following that first report, the same group expanded their study and randomized 32 patients who receive IFLT versus 33 patients who received conventional liver transplant (LT).2 The use of the IFLT demonstrated a significant reduction in early allograft dysfunction (EAD): 6% for the IFLT group versus 24% for conventional LT (P = .044). Nonanastomotic biliary strictures at 12 months were significantly reduced as well (8% for IFLT vs 36% for conventional LT; P = .014). This study demonstrated the feasibility and safety of IFLT procedures to avoid complications related to IRI. However, we believe that the greatest potential benefit of IFLT is for livers from extended criteria donors, especially steatotic donor livers.3 In the previous study cohort, based on IFLT and conventional LT groups, only 19% and 14% of the donors, respectively, met extended criteria. Notably, no donor presented with severe macrovesicular steatosis (>60%), and only 6% in the IFLT group and 9% in the conventional LT group presented with moderate steatosis (30%-60%).2
Case Report
The IFLT technique was introduced at our institution in January 2024. This is the first case of IFLT reported in a Western country. The donor was a 63-year-old male patient who had died of head trauma after a 2-day stay in the intensive care unit. The donor’s body mass index (calculated as kilograms body weight per meter squared) was 44.46, and liver ultrasonography demonstrated severe steatosis. The recipient was a 58-year-old female patient with polycystic liver disease, chronic liver failure, decompensated ascites nonresponsive to diuretics, a Child-Pugh of score 10, a sodium Model for End-Stage Liver Disease score of 26, and a body mass index of 18.21.
Donor procedure
The donor procedure consisted of multi-organ procurement, including the liver and both kidneys. Liver procurement took 8 hours. Donor liver ischemia-free procurement was completed by using a stepwise technique via complete mobilization of the liver and retrohepatic vena cava (Figure 1A), full dissection of pedicle vascular structures, and isolation of the celiac trunk and splenic artery.
A cannula was inserted into the splenic artery, and 2 other cannulas via an interposition vein graft (external iliac vein graft) were placed into the portal vein and the inferior vena cava (IVC). The portal vein and hepatic artery cannulas were connected to the corresponding lines of the normothermic machine perfusion (NMP) device, whereas the vena cava cannula was attached to the organ reservoir. A tube was placed in the biliary duct to control bile production.
Finally, the celiac trunk, gastroduodenal artery, and portal vein bifurcation were closed and divided. The NMP was started; meanwhile, the suprahepatic and infrahepatic vena cava were clamped and divided. Simultaneously, the infradiaphragmatic aorta was clamped, and classic kidney cold perfusion was started.
The liver remained connected to the device for 3 hours before the recipient procedure. Graft biopsy showed >60% of macrovesicular steatosis. Assessment of the procured liver during ex situ NMP showed production of 35 mL of bile, a reduction of lactate from 5.9 mmol/L to 1.8 mmol/L, and a reduction of alanine transaminase from 330 U/L to 115 U/L; pH value of perfusate was 7.5.
Recipient procedure
Liver implant (Figure 1B), after diseased liver removal, was performed with the caval replacement technique with venovenous bypass, and in situ NMP was maintained until liver revascularization.
Caval stumps were clamped, and a graft was moved into the abdominal cavity. During the entire process, twisting of perfusion lines was avoided, and a perfusionist controlled an adequate and continuous blood supply to the graft. Procedures were performed as follows. End-to-end suprahepatic IVC and portal vein anastomoses were performed. Arterial reconstruction occurred next, followed by infrahepatic IVC reconstruction. Portal and arterial clamps were opened, and simultaneously the portal cannula was closed to allow liver revascularization and in situ NMP was stopped. Before superior caval clamp removal, a flush through the caval cannula was performed to wash out the perfusion solution. Then, sequentially, the caval cannula was closed, the superior vena cava clamp was opened, and the IVC clamp was opened after stopping venovenous bypass. Portal and caval interposition grafts were divided with vascular linear stapler.
Perioperative outcomes
Recipient recovery was uneventful, the stay in the intensive care unit lasted 7 days, and overall hospitalization lasted 28 days. On postoperative day 1, the peak for aspartate transaminase was 1095 U/L, and the peak for alanine transaminase was 816 U/L. On postoperative day 7, total bilirubin was 1.01 mg/dL, and the international normalized ratio was 1.20. Standard institutional post-LT care and immunosuppression were provided.
Technical considerations
For this first case, we opted for caval replacement technique, but the so-called piggyback caval reconstruction technique is possible by either a terminolateral or a laterolateral approach. Attention is crucial to ensure correct venous outflow.
When possible, an arterial cannula should be placed in the gastroduodenal artery to ensure uninterrupted arterial perfusion during the arterial anastomosis procedure. The choice depends on arterial diameter and length; alternatively, as in our case, the splenic artery can be used.
A technical difference with descriptions of the IFLT technique from previous reports1,2 was the use of a caval interposition graft instead of cannula placement directly into the inferior caval stump. In our opinion, this technical difference does not reasonably increase the complexity of the intervention, but rather facilitates the completion of all vascular anastomoses, including the IVC anastomosis under NMP, and pro-vides better outflow control during liver implantation. Moreover, a laterolateral caval anastomosis is eventually possible when the piggyback technique is used, and this option may be more relevant when the recipient hepatocaval confluence clamp in the terminolateral anastomosis is hemodynamically not tolerated.
Discussion
The IFLT method is an appealing procedure, and the ability to abolish IRI identifies a potential advantage of this technique to reduce LT complications and enhance recovery of graft function.2,5
Organ shortage and wait list mortality have remained important problems for LT surgeons. Therefore, IFLT may help relieve the chronic organ shortage by improving the viability of expanded criteria liver donors, particularly for donors with moderate to severe steatotic livers. Nonalcoholic fatty liver disease is expected to become more frequent among liver donors. Presently, nonalcoholic fatty liver disease has an overall global prevalence of 30% and prevalence of 25% in Western Europe.4 Moderate to severe steatotic livers are more susceptible to IRI. Furthermore, steatosis >30% has been demonstrated to worsen post-LT incidence of EAD, primary nonfunction, biliary complications, and patient and graft survival.3
Consequently, acceptability of donors with fatty liver disease remains controversial for LT surgeons; presently, such grafts are often discarded. Reduction of cold ischemia time seems to decrease EAD and improve graft survival, especially in fatty livers.2,3 Avoidance of cold ischemia time and IRI is probably a crucial aspect to improve outcomes in this context. Retrospective analysis of the aforementioned group from China showed that IFLT use in moderate to severe steatotic liver cohorts can reduce EAD incidence and that IFLT could be an independent factor of post-LT survival.5 The favorable outcome in our recipient who received a donor liver with >60% macrovesicular steatosis correlates with the previously published data.
Presently, the evidence is insufficient to definitively support these arguments. Nevertheless, our obser-vations legitimize and support the design of future randomized multicenter trials. However, transplant teams face several limitations, including technical and organizational obstacles. The IFLT procedures require further simplification to demonstrate technical viability for the less-experienced surgeons on a transplant team who are likely to be assigned the task of liver procurement. Another aspect worthy of improvement is the prolonged intervention time, the reduction of which could improve the timing of organ procurement.
For now, use of IFLT is limited to local donors as a result of factors such as the cumbersome mobility/transport of the NMP system; multicenter implementation will require development of nimble NMP devices, as well as properly trained surgical teams and perfusionists. Moreover, a backup cold preservation method should be readily available in case of IFLT procedure failure during procedure or transfer.
Despite the fascination evoked by IFLT, this is, to our knowledge, the first Italian and European report to be published on IFLT. To date, only one other center has used the IFLT technique, and our successful attempt meets the need to externally validate the feasibility of this technique.
The IFLT technique is technically demanding, and its organization requires planning, which may explain the lack of broad, multi-center adoption. In order to overcome these difficult aspects, further studies are needed. However, we strongly believe that the potential advantages make the IFLT technique worthy of being improved and adopted.
References:
- He X, Guo Z, Zhao Q, et al. The first case of ischemia-free organ transplantation in humans: a proof of concept. Am J Transplant. 2018;18(3):737-744. doi:10.1111/ajt.14583
CrossRef - PubMed - Guo Z, Zhao Q, Jia Z, et al. A randomized-controlled trial of ischemia-free liver transplantation for end-stage liver disease. J Hepatol. 2023;79(2):394-402. doi:10.1016/j.jhep.2023.04.010
CrossRef - PubMed - Patrono D, De Stefano N, Vissio E, et al. How to preserve steatotic liver grafts for transplantation. J Clin Med. 2023;12(12):3982. doi:10.3390/jcm12123982
CrossRef - PubMed - Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335-1347.
doi:10.1097/HEP.0000000000000004
CrossRef - PubMed - Chen M, Chen Z, Lin X, et al. Application of ischaemia-free liver transplantation improves prognosis of patients with steatotic donor livers: a retrospective study. Transpl Int. 2021;34(7):1261-1270. doi:10.1111/tri.13828
CrossRef - PubMed

Volume : 22
Issue : 8
Pages : 650 - 653
DOI : 10.6002/ect.2024.0180
From the 1Department of Hepatobiliary and Pancreatic Surgery and Organ Transplantation, and the 2Anesthesiology and Intensive Care Unit, University Hospital San Martino, Genoa, Italy
Acknowledgements: The authors have not received any funding or grants in support of the presented research or for the preparation of this work and have no declarations of potential conflicts of interest.
Corresponding author: Marco Miggino, HBP Surgery and Organ Transplantation Department, University Hospital San Martino, 16132 Genoa, Italy
Phone: +39 010 555 5857
E-mail: marco.miggino@hsanmartino.it
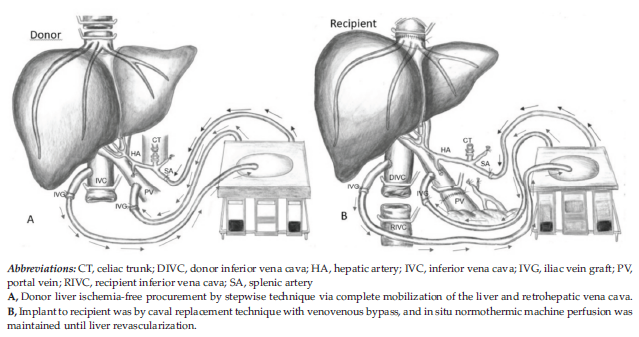
Figure 1. Donor Liver Ischemia-Free Procurement for Transplant