Volume: 19 Issue: 6 June 2021
FULL TEXT
Abstract
Objective: In children who undergo renal transplant, vesicoureteral reflux on the transplanted kidney is a serious complication that may result in organ loss. In this study, we aimed to evaluate the results of endoscopic and open surgical techniques in the treatment of patients with recurrent urinary tract infections and vesicoureteral reflux after renal transplant. Material and Methods: The files of pediatric patients who underwent renal transplant in our hospital between January 2016 and January 2019 were evaluated retrospectively. In this single-center analysis, we investigated the incidence of vesicoureteral reflux in the kidney transplant recipients and the results of various approaches to treat it. Results: Eighty pediatric patients underwent renal transplant between January 2016 and January 2019. Fourteen of those patients (17.5%, 7 female and 7 male) were diagnosed with vesicoureteral reflux in the postoperative period. Twelve of 14 patients received endoscopic injections as the first treatment. Clinical or radiological success was achieved in 5 patients (5 of 15 injection treatments, 33%); in 4 patients (4/12, 33.3%) success was after the first endoscopic injection treatment, and in 1 patient (1/3, 33.3%) success was after the second injection. Meanwhile, clinical or radiological success was achieved in 6 of 7 patients who underwent redo ureteroneocystostomy (6/7, 85.7%). Conclusion: Although symptomatic vesicoureteral reflux after renal transplant is rare in pediatric patients, it is an important cause of morbidity as it requires recurrent surgical procedures. Although endoscopic treatment is safe and minimally invasive, the success rate is lower than expected, and redo of ureteral reimplant may be required in most cases.
Key words : Endoscopic injection, Kidney transplant, Ureteroneocystostomy, Subureteric transurethral injection
Introduction
Renal transplant is the ideal treatment for pediatric patients with end-stage renal disease (ESRD) and avoids the complications of dialysis, offers a better and more comfortable life opportunity, as well as more favorable conditions for healthy growth and development.1,2
The incidence of vesicoureteral reflux (VUR) after renal transplant in pediatric patients is reported to be as high as 58%.3 Pyelonephritis due to VUR after kidney transplant can be an important cause of morbidity and may lead to organ loss. Although many surgeons perform antirefluxive ureteroneocystostomy (UNC) in pediatric recipients during transplant, the risk of VUR remains high and treatment options remain unclear.4
Although a primary surgical approach is often used in the treatment of VUR in kidney transplant patients, endoscopic treatment with injection material composed of dextranomer and hyaluronic acid copolymer (Dx-HA) has become an alternative method with a success rate of 40% to 57%.5-8 Although repeat UNC has traditionally been the gold standard treatment for VUR, this operation is associated with high morbidity and remains a difficult procedure because of the possibility of insufficient arterial support of the transplanted ureter; also, repeated surgeries are associated with higher likelihood of severe scar tissue.7,8 Treatments of posttransplant VUR with prophylactic antibiotics or endoscopic Dx-HA injection are more acceptable options that may be applied before the more difficult option of redo UNC surgery.8,9 In this study, we present a retrospective evaluation of treatment methods and outcomes of VUR after renal transplant.
Materials and Methods
This study was conducted in accordance with the Declaration of Helsinki and with the approval of our institutional ethics committee (January 8, 2020; Decision No. KAEK-15). Written informed consent was obtained from the parents of all participants.
A transplant team of general surgeons performed kidney transplant procedures on a total of 463 pediatric patients at our Faculty of Medicine Hospital from July 20, 1994, to March 5, 2020. Because the members of the urology team who treated the urological complications during this period and the members of the organ transplant surgery team were all completely changed over the span of 16 years, we chose to limit our study to include only the data of a total of 114 pediatric patients who were treated by the most recent surgical teams between January 2016 and March 2020. Of these 114 patients, 80 children with at least 1 year of follow-up were included in the study. Fourteen of those patients were diagnosed with VUR, and 13 who required interventional treatment were treated by the urology team (Table 1).
Kidney transplant technique
In our hospital, the pediatric kidney transplant team consists of general surgeons only. Ureterovesical anastomosis was performed during transplant according to Lich-Gregoir technique in all cases. A double J stent was routinely inserted into the ureter to protect the anastomosis and to allow it to heal more quickly. In the third postoperative week, the urology team used endoscopy to remove the double J stents.
Posttransplant follow-up protocol
All patients were followed up at the renal transplant clinic with routine clinical visits. Recurrent febrile urinary tract infections (UTI) were defined as UTI symptoms with 3 or more episodes within 1 year, diagnosed as fever greater than 38 °C and documented growth in the urine culture of more than 100?000 colony-forming units/mL. Voiding cystourethrography (VCUG) was not performed in any patient after transplant unless the following main indications were present: recurrent febrile UTI or the presence of hydronephrosis that showed progression and/or caused an increase in serum creatine levels. The patients’ VUR ratings were classified according to the International Reflux Study classification system.10
Each patient diagnosed with VUR was first examined for the presence of bladder-bowel dysfunction, and conservative treatments were initiated as needed, which included voiding timing, fluid intake, constipation treatment, and antibiotic prophylaxis. Interventional treatment was started in patients whose VUR did not improve and in whom clinical responses could not be obtained after conservative treatment.
Surgical technique (subureteric injection)
The patients received Dx-HA (Dexell, Istem Medikal) via a subureteric transurethral injection (STING) with a 3.7F Williams needle (Cook) and a 9.5F rigid cystoscope (Storz). Injection at the 6-o’clock position of the ureteral orifice was continued until a satisfactory volcano shaped peak was achieved around the orifice. Intraureteral injection was performed when needed with the previously described technique.11 During the endoscopic treatment, if the angle between the cystoscope and the ureteral orifice did not allow for an effective injection or an adequate coaptation was not observed in the ureteral orifice despite the injection or if the submucosal tunnel was considered to be rather short during endoscopy, then the patient was no longer considered a candidate for injection therapy.
On the first postoperative day and first week after endoscopic treatment, ultrasonography of the urinary system and serum creatinine were measured to detect possible ureteral obstruction. Urinary system ultrasonography, serum creatinine, estimated glomerular filtration rate (eGFR), and urine culture were performed monthly as a follow-up protocol.
Surgical technique (redo ureteroneocystostomy)
If the endoscopic treatment had failed (the presence of recurrent febrile UTI or increased creatine values due to persistent effect of hydronephrosis), then a redo UNC was performed with a dual intravesical and extravesical approach, as described by Paquin.12 The length of the prepared tunnel was at least 5 times the diameter of the ureter. Double J stents of appropriate size were placed in the ureter and removed endoscopically, under sedation, 3 weeks after the procedure.
The success rates for Dx-HA injection and redo UNC were assessed separately in terms of radiological and clinical success. Radiological success was defined as postoperative VUR grade between 0 and 1, whereas clinical success was defined as no febrile UTI during follow-up.
Data from this single-center study were analyzed retrospectively. We evaluated the rate of posttransplant VUR occurrence, the effects of treatment on eGFR values and rates of graft survival for patients with VUR, and the efficacy and safety of surgical treatments.
Results
Eighty pediatric patients underwent renal transplant at our center between January 2016 and January 2019. The mean age of the patients at the time of the transplant was 14 years (range, 1-18 years). The rate of deceased donor kidney transplant was 41.25%. The relationships between recipients and living donors were as follows: 25% of living donors were mothers of the recipients, 22.5% were fathers, 3.75% were siblings, and 7.5% were other relatives (ie, grandparent, uncle, or aunt). Each patient underwent detailed urological evaluation. In addition, functional examination of the bladder with video urodynamics was performed in patients with posterior urethral valve and neurogenic bladder (patients 3, 4, 5, and 13). Clean intermittent catheterization treatment was recommended for 1 patient (patient 4) and clean intermittent catheterization with anticholinergic treatment for another (patient 13). In the posttransplant period, 18 VCUG examinations were performed and VUR was detected in 14 patients (14/80; 17.5%). Thirteen of these 14 patients (6 females and 7 males, 16.2%) required interventional treatment. The remaining single patient’s recurrent UTI was controlled by conservative treatment and required only observation. The demographic data and clinical features of the patients diagnosed with VUR are summarized in Table 2.
All but 1 of the patients who were diagnosed with VUR were treated with STING as the initial treatment. The remaining patient (patient 13) underwent redo UNC because the ureteral orifice was found to be unsuitable for injection during cystoscopy. The procedures for 4 of the 12 patients who underwent injection therapy (patients 2, 3, 10, and 12; ie, 33.3%) were considered clinically or radiologically successful after the first STING session. A second STING session was applied to 3 of 8 patients (patients 1, 6, and 9) whose first injection had failed. An asymptomatic patient (patient 6) whose reflux persisted radiologically after the second STING treatment but whose UTI did not relapse is presently under treatment with antibiotic prophylaxis. Although VUR disappeared in 1 of 2 patients who received the second injection (patient 9), the other patient (patient 1) underwent redo UNC because the second STING treatment was not successful. Four of 5 patients (patients 4, 5, 7, 8, and 11) showed good clinical responses to redo UNC after unsuccessful first STING injections. However, UTI recurred in the remaining patient after redo UNC treatment. In total, clinical responses were obtained in 5 of 15 injection treatments, and the success rate was 33.3%. Adequate clinical responses were obtained in 6 of 7 patients (85.7%) who underwent redo UNC; therefore, this treatment approach was deemed successful (Figure 1). The duration of the follow-up with conservative treatment until the first surgical intervention of patients diagnosed with VUR was 5.3 months (range, 2-12 months), and the mean follow-up time after the final surgical procedure was 27.6 months (range, 7-51 months). The eGFR values that were recorded during these follow-up periods are presented in Table 3.
Patient 2 showed low eGFR values before the surgical interventions, and although the VUR disappeared after STING treatment, the graft rejection could not be prevented at postoperative month 10. Patient 3 presented with increased creatinine levels and grade 2 hydronephrosis (according to the Society for Fetal Urology classification system) after the first STING injection and required double J stent placement to address a prediagnosis of obstruction secondary to the injection. After the stent was placed, the patient’s serum creatine values returned to normal and did not rise again after the stent was removed. Another patient who underwent redo UNC developed a postoperative urinary extravasation complication. The patient was treated with a catheter, and the extravasation healed after 3 weeks.
Discussion
The ESRD etiology in the pediatric population appears to be a factor for treatment, postoperative complications, and outcomes before and after transplant.13 Incidence of congenital anomalies of the kidney and urinary tract (CAKUT) comprises 50% of the etiology of pediatric ESRD.13 In our study, CAKUT was the most frequent cause of the ESRD, with an incidence of 37.5%. High CAKUT rates require detailed evaluation of the entire urinary system before transplant. In addition, graft survival and complication rates in transplant recipients with CAKUT etiology are more curious. Jahromi and colleagues13 have suggested that pediatric patients who require transplant to treat urological problems have no disadvantage for graft survival compared with patients with ESRD of other origin. Barry14 has reported several treatment options that may achieve a leak-free bladder structure with low pressure, capable of complete voiding, in patients with abnormal bladder who would otherwise store urine at high pressure, with leakage, and who typically remain incapable of complete voiding via natural urination in the pretransplant period. Therefore, knowledge and management of these bladder modifications should facilitate successful transplants in patients with abnormal bladders and produce favorable outcomes that match or approach the success rates achieved in patients with a normal bladder structure. Surprisingly, Herthelius and Oborn have reported that functional bladder disorders do not increase the incidence of UTI after transplant.15 Patients with transplant VUR who do not develop febrile UTI may be successfully treated with bladder training and temporary antibiotic prophylaxis.16 In our study, all patients who developed reflux underwent bladder evaluations regardless of the presence of UTI. Patients were followed conservatively with behavioral therapy for an average of 5.3 months with expectation that VUR would improve. However, except for 1 patient with a low reflux level, no other patient benefited from conservative treatment. The reason for this lack of benefit may be that most of our patients had high-level, symptomatic reflux.
Posttransplant incidence rates for VUR in adult and pediatric recipients vary between 1% and 86%.3-5 In our study, the incidence of VUR after renal transplant in pediatric patients was 17.5%, which is similar to rates reported by Sheth and colleagues.8 Ranchin and colleagues3 have routinely performed VCUG in patients after transplant and have reported incidence rates of VUR for the pediatric kidney transplant population as high as 58%. In this study, we used VCUG to investigate the presence of VUR in patients with UTI concomitant with fever and hydronephrosis, which may cause renal damage.
Many factors may affect the outcome of Lich-Gregoir technique, such as submucosal tunnel length, bladder wall quality, stent use, and surgeon experience.17,18 Tunnel length at the time of transplant is also very important. Neuhaus and colleagues18 have observed submucosal tunnels shorter than 1 cm in all patients who developed VUR after transplant. They also observed that submucosal tunnel length of least 3 cm was associated with complete disappearance of reflux. Although different open surgical methods have specific advantages and complications, these open methods all have the common goal to lengthen the intramural portion of the ureter by placement in the submucosal area and creation of a suitable submucosal tunnel and a robust detrusor support. All of these techniques have been deemed safe, with low complication rates and excellent success rates (92%-98%).19 We also suggest that the length of the tunnel in transplant may play a role in postoperative VUR; unfortunately, because of the retrospective nature of our study, we did not have access to data regarding submucosal tunnel lengths or the bladder wall qualities.
Although the association of VUR, UTI, and reflux nephropathy has been established, the clinical significance of posttransplant VUR remains controversial. Another important point of discussion is the effect of treatment of posttransplant VUR on eGFR values and graft survival. Although no published studies have shown that VUR treatment may correct eGFR, the studies that do exist have most often reported on treatment of patients with high-grade reflux (grade 4-5); therefore, there is as yet no basis to consider long-term results.16,20 The result of our present study do not prove that posttransplant treatment of high-grade VUR improves eGFR values; however, we have shown evidence that posttransplant treatment of high-grade VUR may prolong graft survival and increase the patients’ quality of life by reduction of UTI frequency.
The most important reason for correction of VUR after transplant may be to mitigate the risk of premature graft loss due to symptomatic UTI.11 Studies have shown that posttransplant UTI may lead to a higher rate of graft loss.13 Preferred surgical treatment options include endoscopic STING therapy, as well as open surgical ureteral reimplant, which has a higher risk of graft loss due to severe complications such as stenosis, fistula, or ureteral necrosis.
Studies with adult patients have shown that endoscopic STING treatment is preferred over an open surgical approach for treatment of posttransplant VUR because of the low complication rates, low risk of graft loss, and high success rate. Success rates of different bulking agents such as Dx-HA, collagen, and Teflon have been compared, and rates up to 53% have been reported in adult patients.21 Of these agents, only Dx-HA has been used in both adult and pediatric patients. The success rates of STING with Dx-HA have been reported to be between 53.8% and 56.1% in studies that included 26, 11, and 52 adult kidney transplant patients.11,22,23 Williams and colleagues7 reported that among 8 pediatric patients who underwent 9 Dx-HA injections, 4 had complete recession of reflux. The authors stated that the average incidence rate of UTI per patient before the study was 4.4 and no patient had a recurrent UTI at 17.3 months postoperative follow-up.7 Contrary to these data, Sheth and colleagues8 have reported that none of their 11 pediatric patients who underwent injection therapy showed radiological or clinical signs of success; therefore, the authors did not recommend injection therapy as a first-line treatment option for posttransplant VUR. In our study, the total success rate was 5/15 (33.3%) after the first and second endoscopic treatment sessions, and this rate was lower compared with the literature. This low success rate may be explained by the localization of the new ureteral orifice without detrusor support and the high degree of reflux in our cases.
There are many factors that determine the success rate of treatment of primary VUR with STING, but the most important factors are the degree of reflux and achievement of the volcano-shaped peak at the ureteral orifice after injection, which is indicative of a successful procedure.24 Some studies report conflicting results about the intraureteral technique.11,25 Common limitations mentioned in these articles are a low number of patients and/or nonhomogeneity of the study group. We think that an increase in the number of such cases and the subsequent cumulative experience may facilitate higher rates of success. In addition, an intriguing aspect at the beginning of our study was the possibility of etiological determination of those patients who may best benefit from the injection therapy. However, this was not possible because of the low number of patients and low success rates of the injection therapy.
The risk of ureteral obstruction after Dx-HA treatment for primary reflux in pediatric patients ranges from 0.6% to 5.7%.26 Cambareri and colleagues21 described obstruction in 4 patients (2.35%) after 17 Dx-HA treatments. Three of 4 pediatric patients were diagnosed as acute, and 1 patient had obstruction at 1 month after the injection. These 4 patients were treated with double J stent placement, but 2 of the patients required open surgical revision. The authors emphasized that for treatment of posttransplant VUR, obstruction after injection may be more common than expected and may be encountered in the later periods as well. In our series, obstruction due to injection was detected in 1 patient in the early period and was treated with a double J stent.
Although endoscopic injection therapy for treatment of primary reflux is a popular first-line treatment option because of minimal morbidity and rapid recovery, open reimplant surgery remains the gold standard for correction of VUR.27 However, open reimplant surgery can have complications such as poor arterial support of the transplant ureter and various degrees of scar tissue.4,8 Extravesical tunnel-lengthening techniques for correction of posttransplant VUR in both adult and pediatric patients have been described with good results and low rates of complication.28,29 In our series, we achieved a high success rate of 85.7% in the short-term follow-up of pediatric patients who underwent open surgery, and we encountered no serious complications such as ureterovesical anastomosis or organ loss. However, the success of open surgical repair of reflux after transplant may not be as high as in primary reflux.4
The main limitations of our study are the retrospective design, heterogeneous population, small number of cases, and lack of long-term follow-up results. Also, the success rate of injection therapy was presented on the basis of Dx-HA results only. The absence of the polyacrylate-polyalcohol copolymer option, which has been reported to have very high success rates especially in the treatment of primary VUR, is another possible limitation. Cost analyses, which may direct patients and surgeons to a different treatment path, were not performed for the 2 different options that we used for therapeutic purposes.
Conclusions
Symptomatic VUR after pediatric renal transplant is a rare but recurrent type of morbidity that may result in pyelonephritis and potential graft loss. Endoscopic treatment is safe in both adult and pediatric patients, but the success rate is very low. Most patients who underwent endoscopic injection therapy required antibiotic therapy or open redo UNC. We observed that redo UNC treatment was more effective than Dx-HA treatment in pediatric patients, and therefore we suggest that redo UNC should be the first choice in the treatment of posttransplant VUR. Multicenter, comparative, and prospective studies that include large numbers of patients are needed to better understand the factors associated with treatment of posttransplant reflux in pediatric patients.
References:
- Nogueira PC, de Carvalho MF, de Santis Feltran L, Konstantyner T, Sesso R. Inequality in pediatric kidney transplantation in Brazil. Pediatr Nephrol. 2016;31(3):501-507. doi:10.1007/s00467-015-3226-z
CrossRef - PubMed - Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. 2014;371(6):549-558. doi:10.1056/NEJMra1314376
CrossRef - PubMed - Ranchin B, Chapuis F, Dawhara M, et al. Vesicoureteral reflux after kidney transplantation in children. Nephrol Dial Transplant. 2000;15(11):1852-1858. doi:10.1093/ndt/15.11.1852
CrossRef - PubMed - Krishnan A, Swana H, Mathias R, Baskin LS. Redo ureteroneocystostomy using an extravesical approach in pediatric renal transplant patients with reflux: a retrospective analysis and description of technique. J Urol. 2006;176(4 Pt 1):1582-1587; discussion 1587. doi:10.1016/j.juro.2006.06.033
CrossRef - PubMed - Gomez Lujan M, Velarde L, Cruzalegui C, et al. Endoscopic treatment for vesicoureteral reflux in recurrent urinary tract infections in kidney transplant: experience of one center. Transplant Proc. 2018;50(2):513-515. doi:10.1016/j.transproceed.2018.01.007
CrossRef - PubMed - Pichler R, Buttazzoni A, Rehder P, Bartsch G, Steiner H, Oswald J. Endoscopic application of dextranomer/hyaluronic acid copolymer in the treatment of vesico-ureteric reflux after renal transplantation. BJU Int. 2011;107(12):1967-1972. doi:10.1111/j.1464-410X.2010.09792.x
CrossRef - PubMed - Williams MA, Giel DW, Colleen Hastings M. Endoscopic Deflux injection for pediatric transplant reflux: a feasible alternative to open ureteral reimplant. J Pediatr Urol. 2008;4(5):341-344. doi:10.1016/j.jpurol.2008.04.003
CrossRef - PubMed - Sheth KR, White JT, Stanasel I, et al. Comparing treatment modalities for transplant kidney vesicoureteral reflux in the pediatric population. J Pediatr Urol. 2018;14(6):554.e551-554.e556. doi:10.1016/j.jpurol.2018.07.006
CrossRef - PubMed - Hau HM, Tautenhahn HM, Schmelzle M, et al. Management of urologic complications in renal transplantation: a single-center experience. Transplant Proc. 2014;46(5):1332-1339. doi:10.1016/j.transproceed.2014.04.002
CrossRef - PubMed - Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Mobius TE. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol. 1985;15(2):105-109. doi:10.1007/BF02388714
CrossRef - PubMed - Yucel S, Akin Y, Celik O, Erdogru T, Baykara M. Endoscopic vesicoureteral reflux correction in transplanted kidneys: does injection technique matter? J Endourol. 2010;24(10):1661-1664. doi:10.1089/end.2010.0219
CrossRef - PubMed - Paquin AJ Jr. Ureterovesical anastomosis: the description and evaluation of a technique. J Urol. 1959;82:573-583. doi:10.1016/s0022-5347(17)65934-2
CrossRef - PubMed - Jahromi MS, Velasquez MC, Blachman-Braun R, et al. Pediatric kidney transplantation outcomes in children with primary urological abnormalities versus nonurological abnormalities: long-term results. J Urol. 2020;203(2):406-412. doi:10.1097/JU.0000000000000528
CrossRef - PubMed - Barry JM. Kidney transplantation into patients with abnormal bladders. Transplantation. 2004;77(7):1120-1123. doi:10.1097/01.tp.0000116711.59454.f1
CrossRef - PubMed - Herthelius M, Oborn H. Urinary tract infections and bladder dysfunction after renal transplantation in children. J Urol. 2007;177(5):1883-1886. doi:10.1016/j.juro.2007.01.054
CrossRef - PubMed - Wu HY, Concepcion W, Grimm PC. When does vesicoureteral reflux in pediatric kidney transplant patients need treatment? Pediatr Transplant. 2018;22(8):e13299. doi:10.1111/petr.13299
CrossRef - PubMed - Farr A, Gyori G, Muhlbacher F, Husslein P, Bohmig GA, Margreiter M. Gender has no influence on VUR rates after renal transplantation. Transpl Int. 2014;27(11):1152-1158. doi:10.1111/tri.12397
CrossRef - PubMed - Neuhaus TJ, Schwobel M, Schlumpf R, Offner G, Leumann E, Willi U. Pyelonephritis and vesicoureteral reflux after renal transplantation in young children. J Urol. 1997;157(4):1400-1403.
CrossRef - PubMed - Tekgul S, Riedmiller H, Hoebeke P, et al. EAU guidelines on vesicoureteral reflux in children. Eur Urol. 2012;62(3):534-542. doi:10.1016/j.eururo.2012.05.059
CrossRef - PubMed - Fontana I, Ginevri F, Arcuri V, et al. Vesico-ureteral reflux in pediatric kidney transplants: clinical relevance to graft and patient outcome. Pediatr Transplant. 1999;3(3):206-209. doi:10.1034/j.1399-3046.1999.00017.x
CrossRef - PubMed - Cambareri G, Carpenter C, Stock J, Lewis J, Marietti S. Endoscopic antireflux surgery leading to obstruction in pediatric renal transplant patients. Pediatr Transplant. 2017;21(1). doi:10.1111/petr.12838
CrossRef - PubMed - Vemulakonda VM, Koyle MA, Lendvay TS, et al. Endoscopic treatment of symptomatic refluxing renal transplant ureteroneocystostomies in children. Pediatr Transplant. 2010;14(2):212-215. doi:10.1111/j.1399-3046.2009.01196.x
CrossRef - PubMed - Akiki A, Boissier R, Delaporte V, et al. Endoscopic treatment of symptomatic vesicoureteral reflux after renal transplantation. J Urol. 2015;193(1):225-229. doi:10.1016/j.juro.2014.07.103
CrossRef - PubMed - Yucel S, Gupta A, Snodgrass W. Multivariate analysis of factors predicting success with dextranomer/hyaluronic acid injection for vesicoureteral reflux. J Urol. 2007;177(4):1505-1509. doi:10.1016/j.juro.2006.11.077
CrossRef - PubMed - Seifert HH, Mazzola B, Ruszat R, et al. Transurethral injection therapy with dextranomer/hyaluronic acid copolymer (Deflux) for treatment of secondary vesicoureteral reflux after renal transplantation. J Endourol. 2007;21(11):1357-1360. doi:10.1089/end.2007.0020
CrossRef - PubMed - Garcia-Aparicio L, Rodo J, Palazon P, et al. Acute and delayed vesicoureteral obstruction after endoscopic treatment of primary vesicoureteral reflux with dextranomer/hyaluronic acid copolymer: why and how to manage. J Pediatr Urol. 2013;9(4):493-497. doi:10.1016/j.jpurol.2013.02.007
CrossRef - PubMed - Cambareri GM, Hanna MK, Stock JA. Practice patterns among pediatric urologists in the use of Deflux for vesicoureteral reflux: a survey. J Pediatr Urol. 2013;9(6 Pt A):955-961. doi:10.1016/j.jpurol.2013.01.016
CrossRef - PubMed - Dinckan A, Aliosmanoglu I, Kocak H, et al. Surgical correction of vesico-ureteric reflux for recurrent febrile urinary tract infections after kidney transplantation. BJU Int. 2013;112(4):E366-371. doi:10.1111/bju.12016
CrossRef - PubMed - Bouzouita A, Dugardin F, Safsaf A, Sibert L, Pfister C, Grise P. A novel surgical technique for management of vesicoureteral reflux following kidney transplantation: prospective study of 12 cases. Transplant Proc. 2010;42(10):4326-4328. doi:10.1016/j.transproceed.2010.11.001
CrossRef - PubMed

Volume : 19
Issue : 6
Pages : 545 - 552
DOI : 10.6002/ect.2020.0367
From the 1Department of Urology and the 2Department of Pediatric Surgery, the Section of Pediatric Urology, Akdeniz University Faculty of Medicine; and the 3Department of Pediatrics, Section of Pediatric Nephrology, Akdeniz University Faculty of Medicine, Antalya, Turkey
Acknowledgements: The authors have not received any funding or grants in support of the presented research or for the preparation of this work and have no declarations of potential conflicts of interest.
Corresponding author: Murat Uçar, Department of Urology, Section of Pediatric Urology at Akdeniz University Faculty of Medicine, Akdeniz Üniversitesi tip Fakültesi Hastanesi Üroloji Klini?i, Konyaalti/Antalya 07070, Turkey
Phone: +90 505 644 3822
E-mail: drmuratucar@hotmail.com

Table 1. Demographic and Clinical Characteristics of Patients
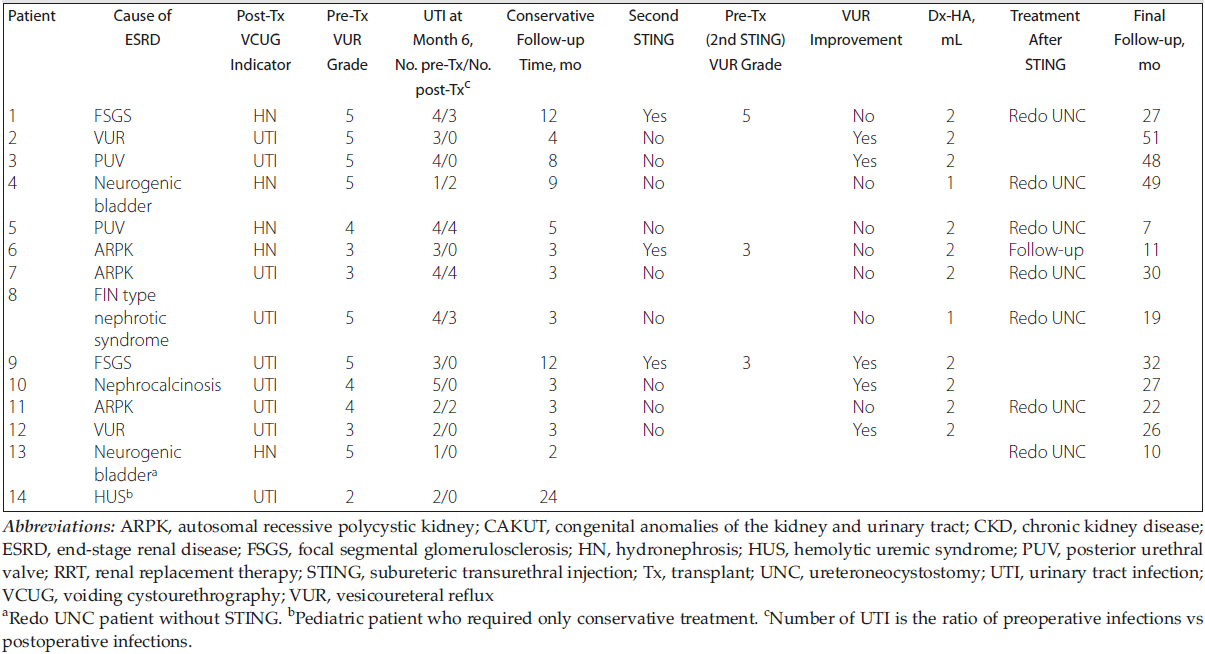
Table 2. Detailed Characteristics of Patients Diagnosed With Posttransplant Vesicoureteral Reflux
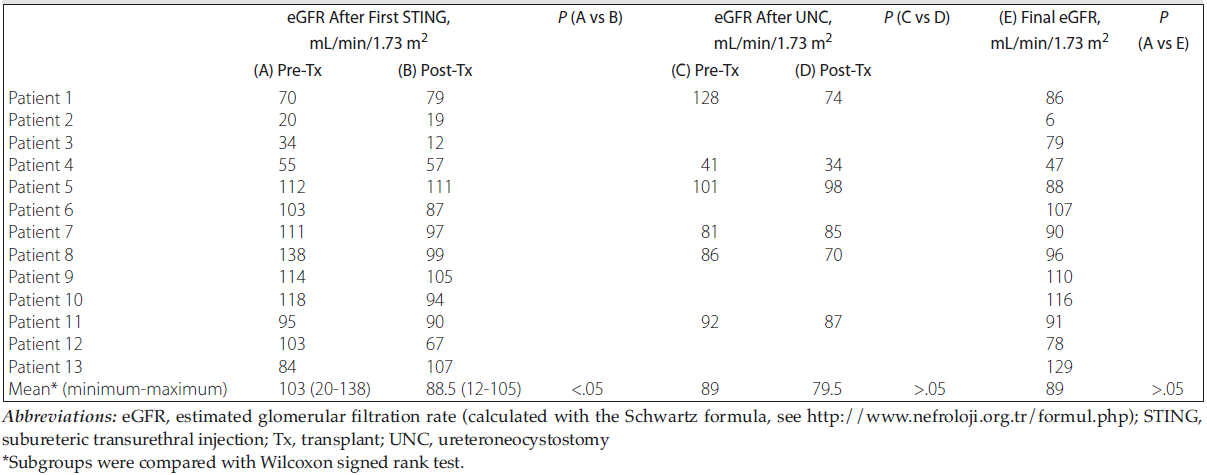
Table 3. Course of Estimated Glomerular Filtration Rate in Patients Before and After Intervention for Posttransplant Vesicoureteral Reflux
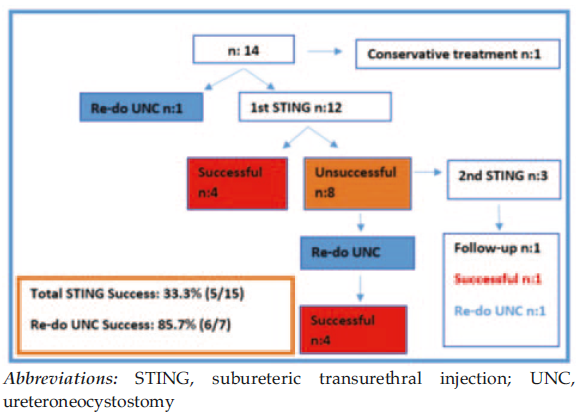
Figure 1. Distribution of Patients Who Underwent Surgical Treatment